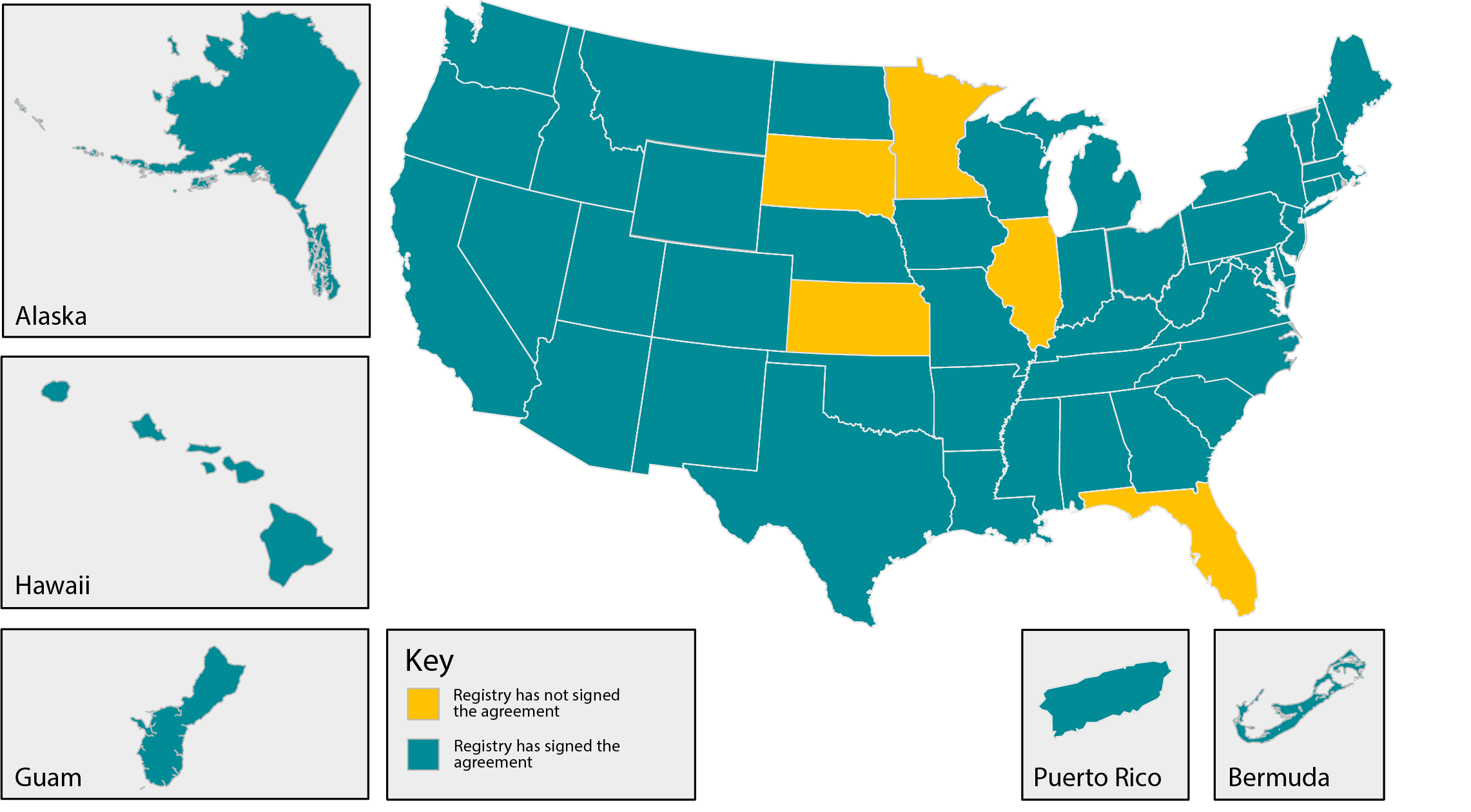
Capturing all newly diagnosed cancer cases is essential to any central cancer registry. Sometimes patients are diagnosed and/or treated in an area that is different from their residence, and agreements must be made between registries to share data. NAACCR has developed a model National Interstate Inter-Registry Data Exchange Agreement which will allow states to exchange data on cases diagnosed or treated in other areas. This single agreement will take the place of multiple inter-registry data exchange agreements. See the list below of the states that have signed the agreement.
- Inter-Registry Data Exchange Agreement – Revised July 2021
Inter-Registry Data Exchange Participants (Last Update August 2023)